From drugdeliverybusiness.com
The diabetes space continues to innovate, and it doesn’t look like that’s stopping any time soon, with 2024 set to be another banner year.At the end of 2023, we compiled a list of the 10 biggest diabetes technology stories of the year. Some of those stories didn’t end in 2023, though, as a few major product launches loom on the horizon.
New CGMs, insulin pumps and combinations of the two will all be coming to the market over the next several months. Here are some of the most highly-anticipated product launches set to take place in the diabetes space in 2024 — plus some of the tech that’s already launched:
Diabetes launches on the horizon
Medtronic’s new pump-sensor combo
In January, Medtronic won CE mark for its MiniMed 780G automated insulin delivery system with the Simplera Sync sensor.
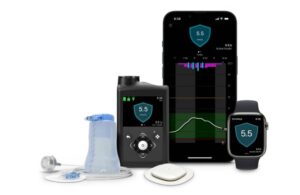
MiniMed 780G, an automated insulin delivery system, offers meal detection technology and provides automatic adjustments and corrections to sugar levels every five minutes. This occurs for both basal (background) and bolus (mealtime) insulin needs. The system already has commercial availability in geographies including the U.S. and Europe in tandem with the Guardian 4 sensor.
Medtronic plans to make MiniMed 780G with Simplera Sync available in Europe through a limited release in spring 2024. It then expects a phased commercial launch in the summer of 2024. While still investigational in the U.S., the combination could still see a stateside launch if clearance comes soon enough.
The company also began rolling out its InPen smart insulin pen system with Simplera in Europe at the end of last year.
Dexcom’s 15-day CGM for non-insulin users
Nearly one year ago, Dexcom launched its next-generation G7 CGM. Now, the company is set for its next major product launch.

At the company’s June Investor Day event, it revealed its plans to bring the new product to market in the U.S. in 2024. According to Dexcom, the target population includes approximately 70% of Americans with diabetes.
The device features a 15-day wear with a cash-pay option. It offers a software experience tailored specifically for non-insulin users. The device won’t “bother” users with the alerts and alarms geared toward insulin users, Dexcom CEO Kevin Sayer told Drug Delivery Business News shortly after the company’s submission to the FDA. Rather, it just offers the insights that users desire.
Stelo exists on the current G7 platform, so Sayer said manufacturing is already in place on the current lines the company has. He also believes the company should get reimbursement for the device over time and create a class of products for the target population.
The company expects to launch Stelo in the U.S. in the summer of 2024.
Insulet’s new pump
Already a leader in the insulin patch pump market with its Omnipod devices — the latest being the Omnipod 5 — Insulet found a way to expand its target population even further.

Omnipod GO, which received clearance for people with type 2 diabetes aged 18 or older, covers the basal-only insulin population. The target population typically takes daily injections of long-acting insulin.
The first-of-its-kind, standalone, wearable insulin delivery system provides a fixed rate of continuous, rapid-acting insulin for 72 hours. It features a tubeless and waterproof pod offered in seven different pre-programmed daily rates. These rates range from 10 to 40 units per day.
Insulet said it developed the product to serve the type 2 diabetes population earlier in their treatment journey. This technology starts them on pod therapy — built on Insulet’s longstanding Omnipod platform — for insulin delivery, rather than daily injections. If a patient becomes insulin-intensive, Insulet offers a seamless transition to another Omnipod product that fits their needs.
The company earmarked a launch for some time in 2024.
These diabetes products already began rolling out this year
Tandem Mobi
Tandem Diabetes Care announced in February that it kicked off the U.S. commercial launch of its Mobi insulin patch pump.
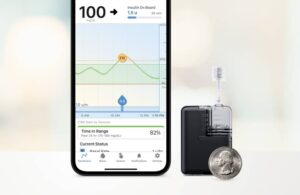
Mobi features a 200-unit insulin cartridge and an on-pump button to provide an alternative to phone control for insulin boluses. It comes in at less than half the size of the existing Tandem pump system, the t:slim X2 pump. Mobi can fit in a coin pocket, clip to clothing or go on the body with an adhesive sleeve.
The system features the same Control-IQ technology that powers the established t:slim X2. Control-IQ, an advanced hybrid closed-loop automated insulin delivery feature, predicts and helps prevent high and low blood sugar. It leads to improved time in range throughout the day and night.
Dexcom ONE+
In Europe, Dexcom has been offering its G6 CGM platform through a differentiated offering called Dexcom ONE.

ONE+ uses Dexcom’s established sensor design, with the company incorporating feedback from users and healthcare professionals to build it. The company said this ensured an easy-to-use, highly effective CGM experience for people treating type 1 or type 2 diabetes with insulin.
This version of the platform utilizes the latest-generation G7 sensor. ONE+ also adds certain notes at certain moments, like meals, insulin administration and sports activities. The system replaces the traditional fingersticks to provide a clear picture of how daily choices affect glucose levels.
CGM/AID pairings

The noteworthy updates include:
- Insulet’s Omnipod 5 with Dexcom’s G7
- Insulet’s Omnipod 5 with Abbott’s FreeStyle Libre 2 Plus
- Tandem Diabetes Care’s t:slim X2 with Dexcom’s G7
- Tandem Diabetes Care’s t:slim X2 with Abbott’s FreeStyle Libre 2 Plus
- Beta Bionics’ iLet with Dexcom’s G7
These diabetes technologies aren’t yet authorized but could be coming
Embecta’s patch pump
Embecta, the BD Diabetes spinoff, has developed a proprietary, disposable pump for people with type 2 diabetes. The company provided analysts with some details on the makeup of the open-loop system earlier this year. It also has a closed-loop version under development to follow. That version features an embedded algorithm that requires Embecta to run a clinical study.
CEO Dev Kurdikar recently told Drug Delivery Business News that the pump “is going to be a vital part” of Embecta’s strategy.
The company in January announced that it submitted the patch pump to the FDA for clearance.
Modular Medical’s insulin pump

Modular Medical designed the 90-day MODD1 with new microfluidics technology to allow for the low-cost pumping of insulin. Its new intuitive design makes the product simple to use and easier to prescribe.
The pump has a reservoir size of 300 units/3mL. Users can monitor the pump activity with their cell phone and do not require an external controller. The pump uses a provided, single-use, disposable battery.
CEO Jeb Besser said the company expects initial questions from the FDA during the quarter ending June 30, 2024. A launch this year may be farfetched but could still loom on the horizon.
PharmaSens’ patch pump

PharmaSens designed its basal-bolus patch pump to combine the ease of an insulin pen with the advantages of a sophisticated pump. It features a 3 mL reservoir, offering extended usage periods and access to reimbursed patch pump therapy. PharmaSens says the design positions Niia Essential as one of the most compact patch pumps on the market.
PharmaSens expects a favourable FDA review and said it is already gearing up for a market launch once that approval comes.
https://www.drugdeliverybusiness.com/these-diabetes-devices-are-set-to-launch-in-2024/
No comments:
Post a Comment