From drugdeliverybusiness.com
It was another banner year for diabetes technology, with regulatory approvals, product launches and M&A all playing their part.
Diabetes technology’s impressive 12 months marked a major trend across medtech as a whole, too.
In 2022, the diabetes space saw plenty of progress from some of its biggest names and this past year proved no different. Big hitters like Dexcom, Abbott, Medtronic, Insulet and more all contributed to some of the biggest stories in 2023. External factors — like popular GLP-1 drugs and big tech names looking to enter the space — also made the cut.
Here are 10 of the most intriguing diabetes technology stories from the past 12 months.
Medtronic was set to buy EOFlow — until it wasn’t
The medtech giant made a big splash in the insulin delivery space when it announced its plan to buy South Korea-based EOFlow.

The deal looked set to rival Medtronic with Insulet and other newer entries to the patch pump space in Tandem Diabetes Care. However, in a surprising turn of events, the deal completely collapsed.
Based upon “multiple breaches,” the company notified EOFlow of its decision to terminate the deal earlier this month. Medtronic offered a statement saying that it still intended to bring a patch pump to the market and could have one in its product pipeline. EOFlow officials also later expressed their belief in the possibility of resurrecting the deal.
It’s possible that the story between the two companies remains unfinished.
Dexcom commences long-awaited G7 launch
The FDA clearance of the Dexcom G7 continuous glucose monitor was one of the major headlines of 2022. Its launch marked one of the biggest stories of 2023.

“When we set out to design G7, our goal was simple: to make the most accurate, easy-to-use CGM available for as many people with diabetes as possible,” said Kevin Sayer, chair, president and CEO of Dexcom. “The approval of Medicare coverage for G7 helps us deliver on that promise.
G7 continued to generate buzz throughout the year, particularly this month. On Dec. 6, Tandem Diabetes Care announced that it launched its updated t:slim X2 insulin pump software with the G7. The integration made Tandem the first to offer automated insulin delivery with the latest-generation CGM.
Just one day later, Beta Bionics became the second to do so, integrating G7 with its iLet bionic pancreas.
Tandem Diabetes Care enters the patch pump fray
As one of the big names in automated insulin delivery already, Tandem Diabetes Care took its portfolio a step further this year.
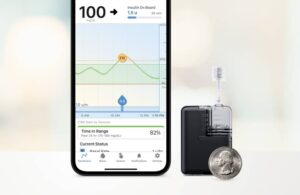
Mobi can fit in a coin pocket, clip to clothing or go on the body with an adhesive sleeve. It also features Tandem’s Control-IQ technology for automated insulin delivery. Tandem said in July that it expects a full launch of the system in early 2024.
Shortly after receiving clearance, Tandem also kicked off a program that provided new and renewing, eligible users of its insulin pump a pathway to its new offerings. Users of the t:slim X2 insulin pump in the U.S. can use the program to move toward owning a Tandem Mobi system.
Abbott and Insulet progress on technology integration
With Dexcom making strides in automated insulin integration, it’s no surprise that its main competitor in the CGM space, Abbott, is doing the same.
Insulet, a leader in insulin patch pump technology, is playing its part in that. The company said in June that it progressed in terms of giving Omnipod 5 — its latest automated insulin delivery system — users a choice over the sensor that accompanies their pump.
Currently, Omnipod 5 works in conjunction with the Dexcom G6 continuous glucose monitor (CGM). Insulet said it expects to soon begin enrolment for a clinical study integrating Omnipod 5 with the Abbott FreeStyle Libre 2 sensor.
This study aims to recruit up to 200 participants with type 1 diabetes, both in the adult and pediatric age groups. It spans the UK, France and Belgium. Insulet said it hopes to demonstrate superior efficacy with Omnipod 5 compared to multiple daily injections.
Medtronic finally picks up approval for the MiniMed 780G
While Medtronic’s attempt to get into the patch pump market fell short of the finish line, the company still had some major wins in insulin delivery this year — namely with FDA approval granted to the next-generation MiniMed 780G automated insulin delivery system with the Guardian 4 sensor.
The FDA approved the device in April and U.S. shipments began in June. It was a long road to get to that point, with analysts suggesting at one point that Medtronic’s Diabetes unit was on the spinoff block. Shortly after picking up approval, the company also fully resolved a longstanding warning letter with the FDA.
In July, the company said that Medicare also covers the MiniMed 780G for all eligible beneficiaries.
Elsewhere, the company’s other insulin delivery offerings progressed, too. Medtronic received CE mark approval for its new Simplera CGM with InPen smart insulin pen integration in September. The all-in-one, disposable CGM seamlessly integrates with the InPen for real-time, personalized dosing guidance to simplify diabetes management.
GLP-1 impact
The GLP-1 drug class has become a major talking point in medtech over the past year and more. This class includes now-household names like Ozempic and Wegovy. The therapeutic class, a glucagon-like peptide 1, has proven to lead to improved blood sugar control and weight loss.
In addition to the popular therapeutics, some companies — like i20 Therapeutics and Vivani Medical — are developing long-term implants that elute GLP-1s.
The question has been asked across medtech and diabetes is no different, especially given that some of these drugs are prescribed to treat the condition — what will be the real impact of GLP-1s? According to some of the biggest names in diabetes, there’s no cause for concern.
BTIG analysts hosted a call with Dr. Osama Hamdy of the Obesity Clinical Program at the Joslin Diabetes Centre to discuss the GLP-1 impact. Hamdy, an Associate Professor of Medicine at Harvard Medical School, sees a minor impact on insulin pumps and a potential boom for CGMs.
An Abbott-sponsored study backed up Hamdy’s opinions. Data from that showed that GLP-1s could be a potential modest accelerator for its FreeStyle Libre CGM product family.
Then there’s Embecta, which doesn’t yet have an insulin patch pump on the market but is intending to enter that space for the type 2 diabetes population. CEO Dev Kurdikar also sees a minor impact on that side of the business, plus he points out Embecta’s other delivery devices, like needles and syringes, could be used to deliver GLP-1s.
Much remains to be seen on the GLP-1 front, but early suggestions should allay any panic in the diabetes device space.
Beta Bionics wins landmark approval
Medtronic and Tandem didn’t have the only major regulatory wins for insulin delivery technology this year. Beta Bionics, before the G7 integration this month, picked up FDA approval for its iLet system in May. The initial clearance covered use with the Dexcom G6.
The Beta Bionics iLet Bionic Pancreas uses an adaptive, closed-loop algorithm. It initializes only with a user’s body weight and requires no additional insulin dosing parameters. The algorithm removes the need to manually adjust insulin pump therapy settings and variables.
iLet also simplifies mealtime use by replacing conventional carb counting with a new meal announcement feature. This feature enables users to estimate the amount of carbs in their meal as “small,” “medium” or “large.” Over time, the algorithm learns to respond to users’ individual insulin needs.
Beta Bionics also received support for its commercialization efforts a few months after the approval came through. The company raised $100 million in August.
Abbott makes a massive diabetes M&A play
While FreeStyle Libre integration continues to progress, Abbott is making moves in other areas of diabetes management.
On Sept. 5 the company entered into a definitive agreement to acquire Bigfoot Biomedical. Less than three weeks later, Abbott completed its buy of the smart insulin management company.
Bigfoot develops the Bigfoot Unity smart insulin management system. The FDA-cleared platform simplifies continuous glucose monitors (CGMs) and the data they produce. The system works exclusively with Abbott’s FreeStyle Libre CGM technology.
It features a smart insulin pen cap, which takes data from a CGM and informs the patient exactly how much insulin they need. Bigfoot Unity also includes a customer smartphone app connected to a cloud-based online portal used by healthcare providers to support patients. The system works with the FreeStyle Libre 2 sensors and all major brands of disposable insulin pens offered in the U.S.
Analysts said they weren’t surprised by the deal and view the strategic tuck-in “favourably,” saying it could accelerate Bigfoot Unity’s adoption for multiple daily injection (MDI) diabetes patients.
Will Apple make its mark in glucose monitoring?
The big names in diabetes plugged along in 2023, but they also received some potential new competition.
Blooomberg reported in April that tech giant Apple has a “moonshot-style” project in the works. According to “sources familiar with the matter,” Apple wants to shake up the CGM market with non-invasive glucose monitoring through the Apple Watch. Bloomberg says the company still has years ahead in this development of the technology, which wouldn’t penetrate the skin.
According to the report, Apple’s project goes back more than 12 years. The company tested the glucose technology on “hundreds of people,” Bloomberg said. That includes human trials of those who don’t know if they’re diabetic, plus those with prediabetes and type 2 diabetes. These tests compared the technology to fingersticks, the report noted.
The initial technology, per the report, comes in the form of a prototype device (around the size of an iPhone) strapped to the bicep. Bloomberg says Apple’s overall goal remains to bring this technology to the Apple Watch.
This also makes CGM an interesting area to watch for potential patent litigation, given Apple’s recent struggles with medical applications for its technology. Both Masimo and AliveCor recently picked up huge victories over the tech giant around medical monitoring technology.
11 diabetes startups you need to know
Between established players and relative newcomers to diabetes technology, plenty of news crossed the airwaves this year. But plenty of companies in their infancy can still make a big difference.
A few noteworthy examples include Diatech Diabetes and its SmartFusion infusion monitoring software. It detects insulin delivery failure and offers insights on how infusion performance affects diabetes management.
Delaware-based Medtech Concept develops a range of medical products for people with diabetes. Its diabetes management system combines digital health software with a market-tested, miniature therapeutic delivery device. The company aims to provide real improvements in the control of diabetes.
Orange Biomed develops its flagship OBM rapid A1c. This platform helps people manage their diabetes through HbA1c testing. The company believes that, despite HbA1c testing’s importance, there remains a lack of at-home monitoring for diabetic patients that offers the accuracy and precision of laboratory devices.
Keep an eye out for these companies and many more as they look to make their mark in the diabetes market.
https://www.drugdeliverybusiness.com/10-biggest-diabetes-tech-stories-2023/
No comments:
Post a Comment